The Lead acid battery is the oldest rechargeable battery with over 150 years of history. However, the conventional battery technology is facing several challenges and there is a great need to modify this technology to meet the current energy need. Lead acid batteries continue to be one of the most widely used rechargeable batteries with around 70% of battery market share worldwide. They have a relatively short life cycle (~500 cycles) and must be replaced frequently resulting in increased replacement costs.
 Whether in hybrid electric vehicles (HEV) or in SLI systems, the battery should be operated continuously in partial-state-of-charge (PSoC). It experiences high charge and discharge process under High-Rate-Partial-State-of-Charge (HRPSoC) cycling duty, when the vehicle braking is heavy.
Whether in hybrid electric vehicles (HEV) or in SLI systems, the battery should be operated continuously in partial-state-of-charge (PSoC). It experiences high charge and discharge process under High-Rate-Partial-State-of-Charge (HRPSoC) cycling duty, when the vehicle braking is heavy.
Lead-acid batteries designed for starting automotive engines are not able to sustain deep discharge. Repeated deep discharge will result in capacity loss and ultimately in premature failure of the battery. This occur due to disintegration of electrodes under continuous mechanical stress, arising from cycling process. Low acid concentration limits the plate activation, promotes corrosion and reduces performance. High acid concentration, on the other hand, raises the open circuit voltage and the battery appears fully charged but provides a low CCA.
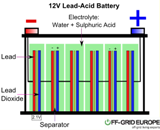 Research in lead-acid batteries is of great interest in developing countries like India and China due to their low manufacturing cost, high material abundance, low temperature performance, low self-discharge rate, easy recycling and higher safety. One of the major limitation of lead-acid battery is its limited cycle life due to sulfation that is caused by the crystallization of non-conductive lead sulfate (PbSO4) crystals, which gradually decreases the amount of usable active material.
Research in lead-acid batteries is of great interest in developing countries like India and China due to their low manufacturing cost, high material abundance, low temperature performance, low self-discharge rate, easy recycling and higher safety. One of the major limitation of lead-acid battery is its limited cycle life due to sulfation that is caused by the crystallization of non-conductive lead sulfate (PbSO4) crystals, which gradually decreases the amount of usable active material.






