Dr. S. S. Verma, Department of Physics, S.L.I.E.T., Longowal, Distt.-Sangrur (Punjab)-148106.

Water crisis
The earth is a water-rich planet, to the tune of about 300 million cubic miles of water, and each cubic mile contains more than one trillion gallons. The water that is not in the oceans is three percent of 300 million cubic miles which is still a lot of water. Unfortunately, most of that three percent is not easily available for our use. Some is tied up in icecaps and glaciers, some is tied up as water vapor in the atmosphere, and the rest is in groundwater, lakes and rivers. The other hard fact is that some of our freshwater supply is simply inaccessible due to its location and depth. The net result is that we make productive use of less than one percent of our global water resources. The problem is that most of that water, approximately 97 percent, is in the oceans which have an average salt content (salinity) of 35,000 parts per million by weight, and drinking that water regularly can kill us. The availability of fresh water is dwindling in many parts of the world, a problem that is expected to grow with populations. One promising source of potablewater is the world’s virtually limitless supply of seawater, but so far desalination technology has been too expensive for widespread use. As the world’s population continues to rise, the pressure on water resources are only going to increase. It is yet to be seen whether technological advances will be able to meet the demand.
Water desalination
As the availability of clean, potable water becomes an increasingly urgent issue in many parts of the world, researchers are searching for new ways to treat salty, brackish or contaminated water to make it usable. Desalination or desalinization refers to any of several processes that remove the excess salt and other minerals from water in order to obtain fresh water suitable for animal consumption or irrigation, and if almost all of the salt is removed, for human consumption, sometimes producing table salt as a by-product.
Developmental status
Significant advances in desalination technology started in the 1900’s and took a major step during WW II because of the need to supply potable water to military troops operating in remote, arid areas. By the 1980’s desalination technology was commercially viable and commonplace by the 1990’s. Today there are more than 16,000 desalination plants worldwide, producing more than 20 billion gallons of drinkable water every day. This is expected to reach more than 30 billion gallons per day by 2020, with one third of that capacity in the Middle East. To put that number in perspective, current global water consumption is estimated to be just less than 1,200 billion gallons. The Middle East has been a leader in desalination so far. Saudi Arabia, United Arab Emirates, Kuwait, and Israel rely heavily on desalination as a source for clean water. Israel gets 40 percent of domestic water from desalination. These countries also have hardly any groundwater or fresh water sources so desalination is a case of innovation by necessity. These countries make up the one percent of the world currently relying on desalination to meet water needs. But the UN predicts that by 2025 14 percent of the world will rely on desalination to meet water needs. Desalination projects can be found in about 150 countries, with many more being planned or under construction. Today’s largest users are in the Middle East – for example, Saudi Arabia derives 50% of its municipal water from desalination and Qatar’s much smaller fresh water supply is entirely from desalination. Currently under construction in Kuwait is a power plant-desalination combined facility that will produce 1.5 GWe and 486,000 cubic meters of fresh water a day.
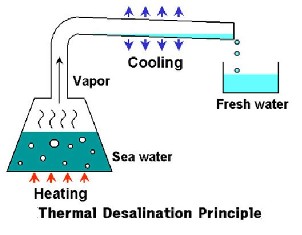
General techniques
There are quite a few technologies today for removing salt from saline water, the oldest being sun-heated water that evaporates and is then condensed, leaving the salt behind. This is also a description of the earth’s hydrologic cycle. The most widely used desalination technologies today are reverse osmosis (RO/60%), multi-stage flash distillation (MSF/26%), and multi-effect distillation (MED/8.2%). Others include electrodialysis, electrode ionization, and hybrid technologies.
Energy requirements (electrical + thermal) for desalinating a range of saline waters, expressed in kWh per cubic meter of fresh water and exclusive of energy required for pre-treatment, brine disposal and water transport, are: RO/3-5.5 kWh; MSF/13.5-25.5 kWH; MED/6.5-11 kWH. Reverse osmosis requires no thermal energy, just mechanical energy to force salty water through a membrane that separates the salt from the water. The laws of physics tell us that the minimum amount of energy required to desalinate seawater is about 1 kWh per cubic meter and under 2 kWh per cubic meter has been achieved in RO, leaving limited opportunities for further reductions.
Limitations
Generally, costs of desalinated water are higher than those of other potable water sources such as fresh water from rivers and groundwater, treated and recycled water, and water conservation. Needless to say, alternatives are not always available and achievable desalination costs today range from $0.5-1 per cubic meter. To put this into perspective, bottled water at $1/liter corresponds to $3,785 per cubic meter. Lastly, salinity levels in oceans are predicted to rise, which would make filtering water more expensive. The more salt there is to filter out, the more energy required. The only way desalination can be a good option to solving the water crisis is if renewable energy is used, costs are lowered, and environmental protections are put in place for marine life too. Companies and countries are trying to lower the amount of energy needed to desalinate water and look into using cleaner energy sources.
Electronics based technologies
Electronics based water desalinations technologies come with many advantages of cost effective, compact, modular, portable, efficient and reliable. Here, some water desalination technologies based on the use of electronic principles are discussed.

Electrochemical mediation:
Chemists at The University of Texas at Austin (USA) and the University of Marburg in Germany have introduced a new method for the desalination of seawater that consumes less energy and is dramatically simpler than conventional techniques. By creating a small electrical field that removes salts from seawater, the process evades the problems confronting current desalination methods by eliminating the need for a membrane and by separating salt from water at a microscale. The technique is called electrochemically mediated seawater desalination and
requires so little energy that it can run on a store-bought battery. To achieve desalination, the researchers apply a small voltage (3.0 volts) to a plastic chip filled with seawater. The chip contains a microchannel with two branches. At the junction of the channel an embedded electrode neutralizes some of the chloride ions in seawater to create an “ion depletion zone” that increases the local electric field compared with the rest of the channel. This change in the electric field is sufficient to redirect salts into one branch, allowing desalinated water to pass through the other branch. The neutralization reaction occurring at the electrode is key to removing the salts in seawater. Like a troll at the foot of the bridge, the ion depletion zone prevents salt from passing through, resulting in the production of freshwater. Right now the microchannels, about the size of a human hair, produce about 40 nanoliters of desalted water per minute. To make this technique practical for individual or communal use, a device would have to produce liters of water per day and the future challenge is to scale up the process.
Shock wave electrodialysis: A team at MIT (USA) has come up with an innovative approach that, unlike most traditional desalination systems, does not separate ions or water molecules with filters, which can become clogged, or boiling, which consumes great amounts of energy. Instead, the system uses an electrically driven shockwave within a stream of flowing water, which pushes salty water to one side of the flow and fresh water to the other, allowing easy separation of the two streams. Membranes in traditional desalination systems, such as those that use reverse osmosis or electrodialysis, are selective barriers which allow molecules of water to pass through, but block the larger sodium and chlorine atoms of salt. Compared to conventional electrodialysis, tThis process looks similar, but it’s fundamentally different. In this process, called shock electrodialysis, water flows through a porous material —in this case, made of tiny glass particles, called a frit — with membranes or electrodes sandwiching the porous material on each side. When an electric current flows through the system, the salty water divides into regions where the salt concentration is either depleted or enriched. When that current is increased to a certain point, it generates a shockwave between these two zones, sharply dividing the streams and allowing the fresh and salty regions to be separated by a simple physical barrier at the center of the flow.
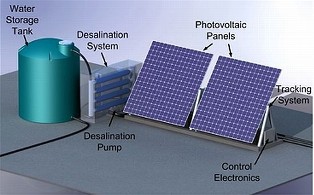
With solar cells:
Modern solar cells, which use energy from light to generate electrons and holes that are then transported out of semiconducting materials and into external circuits for human use, have existed in one form or another for over 60 years. Little attention has been paid, however, to the promise of using light to drive another electricity-generating process — the transport of oppositely charged protons and hydroxides obtained by dissociating water molecules. The researchers write that they have crafted an “ionic analog to the electronic pn- junction solar cell, harnessing light to exploit the semiconductor-like behavior of water and generate ionic electricity. They hope to use such a mechanism to manufacture a device that would directly desalinate saltwater upon exposure to sunlight. In this case, the researchers attained more success by allowing water to permeate through two ion-exchange membranes, one that mostly transported positively charged ions (cations) like protons and one that mostly transported negatively charged ions (anions) like hydroxides, functioning as a pair of chemical gates to attain charge separation. Shining a laser on the system prompted light-sensitive organic dye molecules bound to the membrane to liberate protons, which then transported to the more acidic side of the membrane and produced a measurable ionic current and voltages of over 100 mV in some instances (60 mV on average). Despite crossing the 100 mV photovoltage threshold at times, the level of electric current that the double-membrane system can achieve remains its chief limitation. The photovoltage would need to be magnified by more than another factor of two to reach the ~200 mV mark necessary to desalinate seawater, a target that the researchers are optimistic about hitting. It all comes down to the fundamental physics of how long the charge-carriers persist before recombining to form water. In the long run, desalination is just one possible application of the synthetic light-driven proton pump.
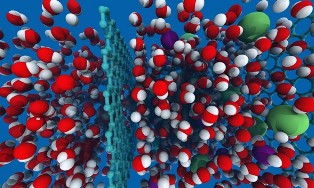
Using graphene sheets:
Graphene sheets with precisely controlled pores have potential to purify water more efficiently than existing methods. When water molecules (red and white) and sodium and chlorine ions (green and purple) in saltwater, on the right, encounter a sheet of graphene (pale blue, center) perforated by holes of the right size, the water passes through (left side), but the sodium and chlorine of the salt are blocked. Reverse osmosis method of water desalination uses membranes to filter the salt from the water but these systems require extremely high pressure — and hence, energy use — to force water through the thick membranes, which are about a thousand times thicker than graphene. The new graphene system operates at much lower pressure, and thus could purify water at far lower cost. Graphene-based system works hundreds of times faster than current techniques, with the same pressure — or, alternatively, the system could run at similar rates to present systems, but with lower pressure. The key to the new process
is very precise control over the size of the holes in the graphene sheet. In addition, the material needed for desalination does not need to be nearly as pure as for electronic or optical uses.
Using batteries: Battery technology could charge up water desalination. The technology that charges batteries for electronic devices could provide fresh water from salty seas as electricity running through a salt water-filled battery draws the salt ions out of the water. The researchers were inspired by sodium ion batteries, which contain salt water. Batteries have two chambers, a positive electrode and a negative electrode, with a separator in between that the ions can flow across. When the battery discharges, the sodium and chloride ions – the two elements of salt – are drawn to one chamber, leaving desalinated water in the other. In a normal battery, the ions diffuse back when the current flows the other direction but researchers had to find a way to keep the salt out of the now-pure water.
Microbial fuel cells: Microbial fuel cells can generate power by cleaning contaminated salty water. The bacteria eat salt and other substances in the contaminated water, and as they clean the water, they generate excess electrons as part of their metabolic processes.
Acknowledgement: The use of information retrieved through various references/sources of internet in this article is highly acknowledged.






